The Class 9 Science and Technology notes are carefully crafted in my own words, based on thorough research and an in-depth understanding of the entire Maharashtra State board textbook of Class 9 Science and Technology. These notes are designed to make complex concepts easy to grasp, helping students gain a solid foundation in science. Whether you’re exploring new topics, revisiting key concepts, or preparing for exams, these notes offer a comprehensive guide to each chapter. With clear explanations and focused summaries, these notes will assist in effective learning and efficient revision, making them a reliable resource for mastering the Class 9 Science and Technology syllabus.
Laws of Motion
Laws of Motion chapter explains the fundamental principles that govern the motion of objects. It introduces concepts like inertia, momentum, force, and acceleration, and explores Newton’s three laws of motion, which form the basis of classical mechanics. These laws explain how objects move under various forces and interactions, offering insights into daily phenomena and practical applications like vehicle dynamics, sports, and machinery.
Motion and Its Types
- Definition of Motion: Motion is the change in position of an object with respect to its surroundings over time.
- Types of Motion:
- Linear Motion: Movement in a straight line (e.g., a car on a straight road).
- Rotational Motion: Movement around a fixed axis (e.g., a spinning top).
- Periodic Motion: Motion that repeats in regular intervals (e.g., a pendulum).
Common Question: How can we observe motion?
Motion is observed by comparing an object’s position relative to a reference point.
Distance and Displacement
- Distance: The total path covered by an object (scalar quantity).
- Displacement: The shortest distance between the starting and ending points (vector quantity).
Example:
If a car travels 5 km east and then 3 km west, the distance is 8 km, but the displacement is 2 km east.
Speed and Velocity
Speed: The rate at which an object covers distance (scalar quantity).
- Formula:

Velocity: The rate of change of displacement with time (vector quantity).
- Formula:
Common Question: Can an object have zero velocity and non-zero speed?
Yes, if an object returns to its starting point, its displacement is zero, making velocity zero, but it still covers a distance.
Acceleration
Definition: The rate of change of velocity with time.
- Formula:
Where u is initial velocity, v is final velocity, and t is time.
- Types of Acceleration:
- Positive Acceleration: Velocity increases over time.
- Negative Acceleration (Deceleration): Velocity decreases over time.
- Zero Acceleration: Velocity remains constant.
Common Question: Why is deceleration also considered acceleration?
Deceleration is a type of acceleration with a negative value, indicating a reduction in velocity.
Newton’s Laws of Motion
First Law (Law of Inertia):
- Statement: An object remains at rest or in uniform motion unless acted upon by an external unbalanced force.
- Inertia: The resistance of an object to change its state of motion or rest.
- Examples:
- A book remains on a table until pushed.
- Passengers lurch forward when a bus suddenly stops.
Common Question: Why is inertia proportional to mass?
Heavier objects have more mass, requiring more force to change their state.
Second Law (Force and Acceleration):
- Statement: The rate of change of momentum of an object is proportional to the applied force and occurs in the direction of the force.
- F=ma
Where F is force, m is mass, and a is acceleration.
- Momentum (P): Product of mass and velocity.
- Formula: P=mv
Common Question: How does Newton’s second law explain a cricket ball hitting a bat?
A faster-moving ball exerts more force due to higher momentum, making it harder to stop.
Third Law (Action and Reaction):
- Statement: For every action, there is an equal and opposite reaction.
- Examples:
- A gun recoils backward when a bullet is fired.
- A rocket propels forward by expelling exhaust gases downward.
Common Question: Why don’t action and reaction forces cancel each other?
They act on different objects, not the same one.
Uniform and Non-Uniform Motion
- Uniform Motion: Equal distances covered in equal intervals of time (e.g., a car at constant speed).
- Non-Uniform Motion: Unequal distances covered in equal intervals of time (e.g., a car in traffic).
Circular Motion
- Definition: Motion along a circular path.
- Uniform Circular Motion: Constant speed but changing direction, causing acceleration.
- Examples:
- The motion of planets around the Sun.
- A stone tied to a string and whirled in a circle.
Formula for Speed in Circular Motion:
Where r is radius and T is time period.
Graphical Representation of Motion
Distance-Time Graphs:
- Uniform Motion: Straight line.
- Non-Uniform Motion: Curved line.
Velocity-Time Graphs:
- Uniform Velocity: Horizontal line.
- Uniform Acceleration: Straight sloped line.
Common Question: What does the slope of a velocity-time graph represent?
The slope represents acceleration.
Equations of Motion
- First Equation (Velocity-Time Relation):
2. Second Equation (Displacement-Time Relation):
3. Third Equation (Velocity-Displacement Relation):
Applications of Newton’s Laws
- Transportation: Seatbelts prevent passengers from moving forward due to inertia during sudden braking.
- Sports: Understanding momentum helps improve performance in games like cricket and tennis.
- Rocket Propulsion: Rockets work on Newton’s third law, expelling gases backward to move forward.
Conclusion:
The chapter “Laws of Motion” lays the foundation of mechanics by explaining how forces influence motion. By mastering these laws, students can understand daily phenomena and solve practical problems in physics and engineering. Concepts like inertia, momentum, and acceleration provide tools to analyze and predict the behavior of moving objects.
Work and Energy
Work and Energy chapter introduces the concepts of work done by forces and the energy transformations that occur during various processes. It explains the mathematical treatment of work, types of mechanical energy (kinetic and potential), the law of conservation of energy, and the relationship between work and energy. Understanding these concepts is crucial for analyzing real-world applications like motion, machines, and power usage.
Work
Definition: Work is said to be done when a force applied to an object causes displacement in the direction of the force.
- Formula: W=F⋅s⋅cosθ
Where F is the applied force, sss is the displacement, and θ is the angle between force and displacement.
Unit of Work:
- SI Unit: Joule (J).
- CGS Unit: Erg.
- Relationship: 1J=107erg.
Example:
If a person applies a force of 50 N to push a box 2 m along a surface in the direction of the force, the work done is:
W=50⋅2⋅cos0∘=100J.
Types of Work
- Positive Work: When force and displacement are in the same direction (θ=0∘).. Example: Lifting an object upward.
- Negative Work: When force and displacement are in opposite directions (θ=180∘). Example: Friction acting on a moving object.
- Zero Work: When there is no displacement or the force is perpendicular to displacement (θ=90∘). Example: Holding a book without moving it.
Student Query: Why is no work done when carrying a heavy bag horizontally?
The force exerted is vertical (against gravity), but the displacement is horizontal, making the angle θ=90∘.
Energy
- Definition: Energy is the capacity to perform work.
- Types of Energy:
- Kinetic Energy (KE): Energy possessed by a body due to its motion.
- Formula:
- Kinetic Energy (KE): Energy possessed by a body due to its motion.
- Where m is mass and v is velocity.
- Example: A moving car or a rolling ball.
2. Potential Energy (PE): Energy possessed by a body due to its position or configuration.
- Formula:
PE=mgh
Where m is mass, g is gravitational acceleration, and hhh is height.
- Example: Water stored in a tank.
Common Question: How are kinetic and potential energy interrelated?
In many systems, such as a pendulum, energy transforms between kinetic and potential while total energy remains constant.
Law of Conservation of Energy
- Statement: Energy can neither be created nor destroyed; it can only transform from one form to another. The total energy in an isolated system remains constant.
Example:
In free fall, potential energy converts to kinetic energy, but the total energy remains the same.
Power
Definition: Power is the rate of doing work.
- Formula:

Where W is work done and t is time.
- Unit of Power:
- SI Unit: Watt (W).
- 1 Watt = 1 Joule/second.
- 1 Horsepower (HP) = 746 Watts.
Example Question: How much power is used to lift a 20 kg weight to a height of 5 m in 10 seconds?
Given W=mgh:
W=20⋅9.8⋅5=980J.
Power:

Relation Between Work and Energy
Work-Energy Theorem: The work done on an object is equal to the change in its kinetic energy.
- Formula:

Example:
If a 2 kg object accelerates from 3 m/s to 5 m/s, work done:

Free Fall and Energy Transformation
- During free fall, potential energy decreases while kinetic energy increases, but total mechanical energy remains constant.
Graph Example: A graph showing the decrease in PE and increase in KE during free fall.
Commercial Unit of Energy
- Definition: The commercial unit of energy is kilowatt-hour (kWh).
1 kWh = 3.6×106J
- Definition: The commercial unit of energy is kilowatt-hour (kWh).
- Application: Used to measure electrical energy consumption in households.
Common Question: How much energy is consumed by a 1.5 kW appliance running for 2 hours?
E=1.5⋅2=3kWh.
Applications of Work and Energy
- Transport: Energy calculations in vehicles for speed and fuel efficiency.
- Machines: Work done by engines, levers, and pulleys.
- Sports: Understanding energy transfer during motion and impacts.
Conclusion:
The chapter “Work and Energy” provides a fundamental understanding of the relationship between force, motion, and energy. By mastering these concepts, students can analyze various phenomena in mechanics, from calculating work done by forces to understanding energy transformations in real-world scenarios. These principles form the cornerstone of physics and its applications.
Current Electricity
Current Electricity chapter explores the concepts of electric charge, electric current, resistance, and the various laws and principles governing them. It delves into practical applications such as electric circuits, domestic electrical wiring, and the role of resistors in controlling current flow. By understanding these principles, students can analyze and design basic electrical systems used in daily life and industries.
Electric Charge
- Definition: Electric charge is a property of matter that causes it to experience a force when placed in an electric field.
- Types of Charge:
- Positive Charge (protons).
- Negative Charge (electrons).
Unit: Coulomb (C).
- Properties of Electric Charge:
- Like charges repel; unlike charges attract.
- Charge is quantized
(q=ne, where e=1.6×10−19C).
- Charge is conserved.
Common Question: Why is charge quantized?
Charge exists in discrete packets, with the smallest unit being the charge of an electron or proton.
Electric Potential and Potential Difference
- Electric Potential: The work done in bringing a unit positive charge from infinity to a point in an electric field.
- Potential Difference: The work done to move a unit positive charge from one point to another.
- Formula:

Where V is potential difference, W is work done, and Q is charge.
Unit: Volt (V).
1 Volt = 1 Joule/Coulomb.
Example: Lightning occurs due to a high potential difference between clouds and the ground.
Electric Current
Definition: Electric current is the flow of electric charges through a conductor.
- Formula:
Where I is current, Q is charge, and t is time.
Unit: Ampere (A).
1 Ampere = 1 Coulomb/second.
Conventional Current vs Electron Flow:
- Conventional current flows from positive to negative terminals.
- Electrons flow from negative to positive terminals.
Common Question: Why is the direction of conventional current opposite to electron flow?
Conventional current is based on the movement of positive charges, established before electrons were discovered.
Resistance and Ohm’s Law
Resistance: The opposition offered by a conductor to the flow of electric current.
- Formula:
- Where R is resistance, V is potential difference, and I is current.
Unit: Ohm (Ω).
- Ohm’s Law: Current is directly proportional to voltage across a conductor, provided its temperature remains constant.
- Formula: V=IR
Common Question: Why do resistors heat up when current flows through them?
Collisions between moving electrons and atoms produce thermal energy, heating the resistor.
Factors Affecting Resistance
- Length of the conductor (R∝L).
- Area of cross-section
3. Material of the conductor (Resistivity ρ).
4. Temperature: Resistance increases with temperature for metals.
Resistivity Formula:
Where ρ is the resistivity of the material.
Electric Circuits
- Definition: A closed path through which current flows, consisting of sources (battery) and components (resistors, wires).
- Circuit Diagram Symbols:
- Battery: + −
- Resistor: _Ω_
- Bulb: ⊙
- Ammeter: A
- Voltmeter: V
Series and Parallel Combination of Resistors
Series Combination:
- Resistors are connected end-to-end.
- Total Resistance:
Rs=R1+R2+R3+…
- Total Resistance:
- Current: Same through all resistors.
- Voltage: Divided across resistors.
Common Question: Why is the effective resistance higher in series?
Each resistor adds to the opposition of current flow.
Parallel Combination:
- Resistors are connected with common endpoints.
- Total Resistance:

- Voltage: Same across all resistors.
- Current: Divided across resistors.
Common Question: Why is the effective resistance lower in parallel?
Parallel paths increase the total area for current flow, reducing resistance.
Electric Power and Energy
Electric Power: The rate at which electrical energy is consumed or produced.
- Formula:
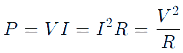
- Unit: Watt (W).
Electric Energy:
- Formula: E=P⋅t
Unit: Kilowatt-hour (kWh).
Domestic Electric Circuits
- Components: Live wire (red), neutral wire (blue), earth wire (green/yellow).
- Safety Measures:
- Fuse: Protects appliances from overcurrent.
- Earthing: Prevents electric shocks.
- Proper insulation and switches.
Applications of Current Electricity
- Electronics: Powering devices like smartphones and laptops.
- Transport: Electric vehicles.
- Industry: Running motors and machinery.
Conclusion:
“Current Electricity” forms the foundation of electrical science, emphasizing the behavior of electric charges, their flow in circuits, and the role of resistance and power. Mastery of these concepts enables students to understand everyday electrical devices and systems, paving the way for advanced studies in physics and engineering.
Measurement of Matter
Measurement of Matter chapter explains the fundamental principles of quantifying substances in chemistry. It covers atomic and molecular masses, laws of chemical combinations, mole concept, valency, and chemical formulae. These concepts are essential for understanding chemical reactions, stoichiometry, and the composition of compounds.
Dalton’s Atomic Theory
- Definition: Proposed by John Dalton, it states that matter is composed of indivisible atoms, and compounds are formed by the chemical combination of different elements in fixed ratios.
- Key Points:
- Atoms of the same element are identical.
- Atoms combine in simple whole-number ratios to form compounds.
Example: Water (H2O) is always composed of 2 parts hydrogen and 1 part oxygen by number of atoms.
Laws of Chemical Combination
Law of Conservation of Matter (Antoine Lavoisier):
- Statement: The total mass of reactants equals the total mass of products in a chemical reaction.
- Example: When 56 g of calcium oxide reacts with 18 g of water, it forms 74 g of slaked lime
(Ca(OH)2)
- Example: When 56 g of calcium oxide reacts with 18 g of water, it forms 74 g of slaked lime
Law of Constant Proportion (J. L. Proust):
- Statement: A compound always contains its constituent elements in a fixed proportion by mass.
- Example: In water, the mass ratio of hydrogen to oxygen is always 1:8.
Verification Experiment:
Copper oxide (CuO) produced by different methods (decomposition of copper carbonate and copper nitrate) always has a mass ratio of copper to oxygen as 4:1.
Atomic Structure
- Components of an Atom:
- Nucleus: Contains protons (+) and neutrons (neutral).
- Electrons: Negatively charged particles in orbits around the nucleus.
- Atomic Size and Mass:
- Atomic radius is expressed in nanometers
(1 nm=10−9 m).
- Atomic mass is due to protons and neutrons in the nucleus.
- Atomic radius is expressed in nanometers
Mole Concept
- Definition: A mole is the amount of substance containing 6.022×1023
(Avogadro’s number) particles. - Formula:

Example:
- Molecular mass of H2O = (2×1)+16=18.
- 18 g of water contains 6.022×1023 molecules.
Valency
- Definition: The combining capacity of an element.
- Types:
- Fixed Valency: Sodium (Na) has a valency of 1.
- Variable Valency: Iron (Fe) exhibits 2 (ferrous) and 3 (ferric).
Electronic Configuration: Valency depends on the number of valence electrons.
Chemical Formulae
- Definition: Representation of a compound using symbols and subscripts.
- Rules for Writing Formulae:
- Identify cation (positive) and anion (negative).
- Cross-multiply valencies.
- Write the cation first, followed by the anion.
Example: Sodium sulfate (Na2SO4)
- Sodium (Na+): Valency = 1.
- Sulfate
(SO2/4–): Valency = 2.
- Formula: Na2SO4
.
Molecular Mass
- Definition: Sum of atomic masses of all atoms in a molecule.
- Example: Molecular mass of H2SO4
:
(2×1)+(1×32)+(4×16)=98 u.
Radicals
- Definition: Charged species formed by the gain or loss of electrons.
- Cations: Positively charged (e.g.,Na+).
- Anions: Negatively charged (e.g., Cl−).
Classification:
- Simple radicals: Na+, Cl−
- Composite radicals:
Importance of the Mole Concept
- Chemical Reactions: Enables accurate measurement of reactants and products.
- Example Calculation:
Find the number of molecules in 36 g of water (H2O).- Molecular mass = 18 u.
- Moles = 36/18=2.
- Molecules = 2×6.022×1023
Applications of Measurement of Matter
- Stoichiometry: Predicts the outcome of chemical reactions.
- Industry: Determines proportions in alloy production and pharmaceuticals.
- Research: Calculates molecular masses in biochemistry and nanotechnology.
Conclusion:
The chapter “Measurement of Matter” provides tools for understanding the quantitative aspects of chemistry. Concepts like the mole, valency, and chemical formulae are foundational for advanced studies in science and engineering. Mastery of these topics allows for precise experimentation and practical application in diverse fields.
Acids, Bases, and Salts
Acids, Bases, and Salts chapter explores the fundamental chemical properties of these three types of substances, their formation, and their role in various chemical reactions. It covers concepts like the Arrhenius theory, pH scale, neutralization, and the properties and uses of acids, bases, and salts. Practical examples and real-life applications help students understand their significance in everyday life and industrial processes.
Acids
- Definition: Substances that produce hydrogen ions (H+) when dissolved in water.
- Examples:
- Strong Acids: Hydrochloric acid (HCl), sulfuric acid (H2SO4).
- Weak Acids: Acetic acid(CH3COOH), carbonic acid (H2CO3)
.
Properties of Acids:
- Sour taste.
- Turn blue litmus paper red.
- React with metals to produce hydrogen gas.
Chemical Reaction Example:
Zn(s)+2HCl(aq)→ZnCl2(aq)+H2(g)
Common Question: Why are acids sour?
Acids release hydrogen ions, which interact with taste receptors, creating a sour taste.
Bases
- Definition: Substances that produce hydroxide ions (OH−) when dissolved in water.
- Examples:
- Strong Bases: Sodium hydroxide (NaOH, potassium hydroxide (KOH).
- Weak Bases: Ammonium hydroxide (NH4OH).
Properties of Bases:
- Bitter taste.
- Turn red litmus paper blue.
- Soapy texture.
Chemical Reaction Example:
Ca(OH)2+CO2→CaCO3+H2O
Common Question: What makes bases feel slippery?
Bases react with skin oils to form soap-like compounds, giving a slippery texture.
Neutralization Reaction
- Definition: Reaction between an acid and a base to form salt and water.
- Example Reaction:
HCl(aq)+NaOH(aq)→NaCl(aq)+H2O(l)
Applications:
- Antacids neutralize stomach acid.
- Soil pH correction using lime (Ca(OH)2)
.
pH Scale
- Definition: A scale to measure the acidity or basicity of a solution, ranging from 0 to 14.
- Acidic: pH < 7.
- Neutral: pH = 7.
- Basic: pH > 7.
Example:
- Lemon juice: pH ≈ 2 (acidic).
- Milk of magnesia: pH ≈ 10 (basic).
Importance of pH:
- Human blood pH is maintained around 7.4 for proper functioning.
- Soil pH affects plant growth.
Salts
- Definition: Ionic compounds formed by the neutralization of an acid and a base.
- Types of Salts:
- Neutral Salts: Formed by a strong acid and a strong base (e.g., NaCl).
- Acidic Salts: Formed by a strong acid and a weak base (e.g., NH4Cl).
- Basic Salts: Formed by a weak acid and a strong base (e.g., CH3COONa).
Example Reaction:
Na2CO3+2HCl→2NaCl+CO2+H2O
Indicators
- Definition: Substances that change color in the presence of an acid or base.
- Types:
- Natural Indicators: Litmus, turmeric.
- Synthetic Indicators: Methyl orange, phenolphthalein.
Color Changes in Indicators:
| Indicator | Acidic Solution | Basic Solution |
|---|---|---|
| Litmus | Red | Blue |
| Phenolphthalein | Colorless | Pink |
| Methyl Orange | Red | Yellow |
Properties of Salts
- Soluble in water.
- Conduct electricity in solution due to ion dissociation.
- Exhibit different colors (e.g., copper sulfate is blue).
Experiment: Test the conductivity of salt solutions using a bulb and circuit.
Electrolysis of Salts
- Definition: Decomposition of a salt solution using electricity.
- Example:
Electrolysis of sodium chloride:2NaCl(aq)+2H2O(l)→2NaOH(aq)+H2(g)+Cl2(g)
Applications:
- Production of chlorine gas.
- Electroplating.
Water of Crystallization
- Definition: Water molecules incorporated into the crystal structure of a compound.
- Examples:
- Blue vitriol: CuSO4⋅5H2O.
- Washing soda: Na2CO3⋅10H2O.
Experiment: Heat hydrated salts to observe water loss and color change.
Applications of Acids, Bases, and Salts
- Acids:
- Hydrochloric acid in cleaning agents.
- Acetic acid in food preservation.
- Bases:
- Sodium hydroxide in soap making.
- Calcium hydroxide in whitewashing.
- Salts:
- Sodium chloride as table salt.
- Baking soda (NaHCO3) in cooking.
Conclusion:
The chapter “Acids, Bases, and Salts” provides a comprehensive understanding of their properties, reactions, and applications. By mastering these concepts, students can appreciate their role in daily life, from household tasks to industrial processes. This foundational knowledge is essential for exploring advanced topics in chemistry.
Classification of Plants
Classification of Plants chapter focuses on understanding the diversity within the plant kingdom by categorizing plants based on specific characteristics. It explores the classification into cryptogams and phanerogams, their sub-divisions, and features of groups such as thallophytes, bryophytes, pteridophytes, gymnosperms, and angiosperms. This classification provides a structured approach to studying plants and their evolutionary relationships.
Kingdom Plantae
- Definition: Kingdom Plantae consists of autotrophic, multicellular organisms with eukaryotic cells that have cell walls.
- Significance: Plants are primary producers, sustaining life by producing food through photosynthesis.
- Basis for Classification:
- Presence or absence of organs like roots, stems, and leaves.
- Conduction tissues for water and food.
- Production and type of seeds (if present).
Common Question: Why are plants considered autotrophic?
They produce their food using sunlight, water, and carbon dioxide through photosynthesis.
Subkingdoms of Plantae
- Cryptogams: Plants with hidden reproductive structures that reproduce by spores.
- Phanerogams: Plants with visible reproductive structures that produce seeds.
Cryptogams
a. Division Thallophyta
- Characteristics:
- Found mostly in aquatic environments.
- Lack true roots, stems, and leaves.
- Autotrophic due to chlorophyll.
- Examples: Algae like Spirogyra, Ulothrix, and Sargassum.
b. Division Bryophyta
- Characteristics:
- Called amphibians of the plant kingdom as they need water for reproduction.
- Lack true roots, stems, and leaves but have root-like rhizoids.
- Reproduce via spores.
- Examples: Moss (Funaria), Marchantia, Riccia.
c. Division Pteridophyta
- Characteristics:
- Have well-developed roots, stems, and leaves.
- Contain vascular tissues for conduction of water and food.
- Reproduce using spores on leaf undersides.
- Examples: Ferns like Nephrolepis, Selaginella, Lycopodium.
Phanerogams
a. Gymnosperms
- Characteristics:
- Woody, perennial, and mostly evergreen.
- Seeds are naked (not enclosed in fruit).
- Male and female flowers are borne on separate sporophylls.
- Examples: Cycas, Pinus, Thuja (Morpankhi).
b. Angiosperms
- Characteristics:
- Seeds are enclosed within fruits.
- Flowering plants with well-developed vascular systems.
- Divided into monocots and dicots based on cotyledons.
Differences Between Monocots and Dicots:
| Feature | Monocots | Dicots |
|---|---|---|
| Cotyledons | One | Two |
| Root System | Fibrous roots | Taproot system |
| Leaf Venation | Parallel venation | Reticulate venation |
| Examples | Maize, Bamboo | Mango, Mustard |
Importance of Plant Classification
- Understanding Evolution: Reveals relationships between species.
- Agricultural Applications: Helps in selecting and improving crops.
- Ecological Studies: Aids in biodiversity conservation.
Conclusion:
The chapter “Classification of Plants” provides a systematic approach to studying plant diversity. By grouping plants based on characteristics such as reproduction, organ development, and seed formation, we gain insights into their evolution and ecological roles. Mastery of plant classification is essential for advancing studies in botany, agriculture, and environmental science.Energy Flow in an Ecosystem
Energy Flow in an Ecosystem chapter explains how energy is transferred among the biotic components of an ecosystem, starting from producers to various levels of consumers and decomposers. It delves into the structure of food chains, food webs, trophic levels, and energy pyramids while emphasizing the unidirectional flow of energy. This chapter also highlights the cycling of nutrients through biogeochemical cycles such as the carbon, nitrogen, and oxygen cycles, demonstrating the balance necessary for ecosystem sustainability.
Ecosystem Overview
- Definition: An ecosystem is a functional unit where living organisms (biotic factors) interact with non-living components (abiotic factors) like air, water, and soil.
- Examples: Forest, pond, desert.
Components:
- Biotic Factors: Producers (plants), consumers (animals), decomposers (fungi, bacteria).
- Abiotic Factors: Sunlight, temperature, water, soil, and nutrients.
Common Question: How do abiotic and biotic factors interact?
Biotic components depend on abiotic factors for survival, such as sunlight for photosynthesis and soil nutrients for plant growth.
Food Chain and Food Web
Food Chain:
- Definition: A linear sequence of organisms where each one serves as food for the next.
- Levels:
- Producers (plants) → Primary Consumers (herbivores) → Secondary Consumers (carnivores) → Tertiary Consumers (apex predators).
Example:
Grass→Grasshopper→Frog→Snake→Eagle
Food Web:
- Definition: An interconnected network of food chains showing multiple feeding relationships.
- Significance: Food webs provide stability to the ecosystem.
Diagram: A simple food web showing interactions among producers, herbivores, and carnivores.
Common Question: Why is a food web more stable than a food chain?
In a food web, organisms have multiple food sources, reducing the impact of the extinction of a single species.
Trophic Levels
- Definition: Each step in a food chain where organisms obtain energy.
- Trophic Levels:
- Producers: Plants that capture solar energy.
- Primary Consumers: Herbivores feeding on plants.
- Secondary Consumers: Carnivores eating herbivores.
- Tertiary Consumers: Apex predators feeding on secondary consumers.
Energy Flow
- Law of Energy Flow: Energy flows in one direction—from the Sun to producers and then to various consumer levels.
- 10% Rule: Only 10% of the energy is transferred to the next trophic level; the rest is lost as heat.
Example:
10,000 kcal(producers)→1,000 kcal(herbivores)→100 kcal(carnivores)
Common Question: Why is energy transfer inefficient?
Energy is lost as heat during metabolic processes and incomplete consumption or digestion.
Energy Pyramid
- Definition: A graphical representation showing energy distribution across trophic levels.
- Characteristics:
- Wide base representing producers.
- Narrow top representing apex consumers.
Decomposers
- Definition: Organisms like fungi and bacteria that break down dead matter into simpler substances.
- Role:
- Recycle nutrients back into the ecosystem.
- Maintain soil fertility.
Example: Decomposition of fallen leaves into humus.
Biogeochemical Cycles
a. Carbon Cycle:
- Definition: The circulation of carbon between living organisms and the atmosphere.
- Key Processes:
- Photosynthesis: Plants absorb CO2.
- Respiration: CO2 is released by organisms.
- Combustion: Burning of fossil fuels adds CO2.
b. Oxygen Cycle:
- Definition: Recycling of oxygen between organisms and the environment.
- Key Processes:
- Photosynthesis: Releases oxygen.
- Respiration: Consumes oxygen.
c. Nitrogen Cycle:
- Definition: Conversion of atmospheric nitrogen into usable forms by organisms.
- Key Processes:
- Nitrogen Fixation: Bacteria convert N2 into ammonia.
- Nitrification: Ammonia to nitrites and nitrates.
- Denitrification: Nitrates to N2.
Human Impact on Ecosystems
- Deforestation: Reduces biodiversity and disrupts nutrient cycles.
- Pollution: Alters the balance of biogeochemical cycles.
- Overexploitation: Affects food web stability.
Common Question: How can we maintain ecosystem balance?
By conserving resources, reducing pollution, and promoting sustainable practices.
Conclusion:
The chapter “Energy Flow in an Ecosystem” highlights the intricate relationships and dependencies within ecosystems. Understanding energy transfer, trophic levels, and nutrient cycles enables us to appreciate the delicate balance necessary for ecosystem health. Conservation and responsible resource management are vital for maintaining this balance and ensuring sustainability for future generations.
Useful and Harmful Microbes
Useful and Harmful Microbes chapter explores the dual role of microorganisms in our daily lives. Microbes, which are too small to be seen with the naked eye, can either be beneficial or harmful. While some microbes help in food production, agriculture, and medicine, others cause diseases in humans, animals, and plants. Understanding their characteristics and applications aids in utilizing their benefits and mitigating their harmful effects.
What are Microbes?
- Definition: Microbes are microscopic organisms, including bacteria, fungi, viruses, protozoa, and algae, that exist in a variety of environments.
- Characteristics:
- Can be unicellular or multicellular.
- Reproduce rapidly through methods like binary fission or budding.
- Found in soil, water, air, and inside other organisms.
Useful Microorganisms
a. Lactobacilli (Bacteria):
- Uses:
- Convert lactose in milk to lactic acid, forming yogurt.
- Used in the production of buttermilk, cheese, and shrikhand.
- Help in making pickles, fermented food, and certain types of bread.
Common Question: Why is yogurt recommended during digestion issues?
Lactobacilli balance gut bacteria, improving digestion and immunity.
b. Rhizobium (Symbiotic Bacteria):
- Role: Fix atmospheric nitrogen into nitrates, enriching soil fertility.
- Application:
- Used in leguminous plants like beans and peas.
- Coated on seeds before sowing to enhance nitrogen fixation.
c. Yeast (Fungus):
- Uses:
- Fermentation: Converts sugar to alcohol and CO₂, used in bread making and brewing.
- Production of ethanol for industrial purposes.
- Bio-remediation: Absorbs industrial toxins like arsenic.
Example Activity: Mix yeast, sugar, and water to observe fermentation.
d. Antibiotics (From Bacteria and Fungi):
- Definition: Substances that kill or inhibit the growth of harmful bacteria.
- Examples: Penicillin (from Penicillium), ampicillin, tetracycline.
- Applications:
- Treat bacterial infections like pneumonia and tuberculosis.
- Narrow-spectrum antibiotics target specific bacteria; broad-spectrum target a variety.
Common Question: Why must antibiotics be taken only on a doctor’s prescription?
Overuse can lead to antibiotic resistance, making bacteria harder to kill.
e. Bio-Remediation Using Microbes:
- Definition: The use of microorganisms to clean environmental pollutants.
- Examples:
- Yarrowia lipolytica removes industrial toxins.
- Alcanivorax cleans oil spills in oceans.
Harmful Microorganisms
a. Clostridium (Bacteria):
- Role: Causes food poisoning by releasing toxins in anaerobic conditions.
- Example Species:
- Clostridium botulinum: Produces the botulinum toxin.
- Clostridium tetani: Causes tetanus.
Prevention: Proper food storage and avoiding anaerobic conditions.
b. Fungi:
- Examples:
- Spoil food items like pickles and jam by releasing mycotoxins.
- Cause spoilage of leather, wood, and fabric in humid conditions.
Common Question: Why should we avoid eating food with fungal growth?
Mycotoxins released by fungi make the food poisonous.
c. Viruses:
- Characteristics:
- Infect living cells to reproduce.
- Cause diseases like AIDS, influenza, and dengue.
Prevention:
- Vaccination and personal hygiene.
- Avoid contact with infected individuals.
Spread and Control of Microbial Diseases
Mode of Transmission:
- Contact: Touching infected individuals (e.g., chickenpox).
- Airborne: Through droplets (e.g., pneumonia).
- Waterborne: Contaminated food and water (e.g., cholera).
- Vector-Borne: Through mosquitoes (e.g., malaria, dengue).
Preventive Measures:
- Vaccination.
- Personal hygiene and proper sanitation.
- Avoiding stagnation of water to control mosquitoes.
Table: Common Microbial Diseases and Prevention
| Disease | Pathogen | Prevention |
|---|---|---|
| Dengue | Virus | Mosquito control, clean surroundings. |
| Cholera | Bacteria | Clean food and water. |
| Malaria | Protozoa | Use of mosquito nets. |
Industrial Applications of Microbes
- Food Industry: Yogurt, cheese, and wine production.
- Pharmaceuticals: Antibiotics and vaccines.
- Bio-fuels: Ethanol production from molasses.
- Agriculture: Biofertilizers using Rhizobium.
Conclusion
Microbes play a significant role in our lives, offering both benefits and challenges. By understanding their characteristics, we can utilize useful microbes for advancements in health, agriculture, and industry while effectively controlling harmful ones to prevent diseases and food spoilage. This knowledge fosters sustainable practices and improves human well-being.
Environmental Management
Environmental Management chapter focuses on the sustainable use of natural resources and strategies to minimize environmental degradation. It emphasizes the importance of weather and climate studies, solid waste management, disaster preparedness, and conservation efforts. The chapter aims to equip students with knowledge and skills to understand environmental challenges and adopt eco-friendly practices to mitigate their effects.
Weather and Climate
Definition of Weather:
- The atmospheric conditions at a specific time and place.
- Examples of Weather Factors: Temperature, humidity, wind speed, and rainfall.
Definition of Climate:
- The average atmospheric conditions of a region over a long period.
Difference Between Weather and Climate:
| Feature | Weather | Climate |
|---|---|---|
| Time Duration | Short-term | Long-term |
| Area Coverage | Local | Regional or global |
| Examples | A rainy day | Monsoon season in India |
Importance of Climate:
- Determines agricultural patterns and crop choices.
- Influences infrastructure development, e.g., runways and bridges.
Meteorology
- Definition: The science studying atmospheric conditions, including storms, rainfall, and climatic patterns.
- Importance:
- Helps in weather forecasting for agriculture, fisheries, and aviation.
- Provides early warnings for natural disasters like tsunamis and cyclones.
Common Question: How does meteorology assist in disaster management?
By predicting extreme weather events, it aids in evacuation and preparation efforts.
Solid Waste Management
Definition of Solid Waste:
Waste materials generated from daily human activities, including organic and inorganic substances.
Types of Solid Waste:
- Domestic Waste: Food scraps, paper, plastics.
- Industrial Waste: Chemicals, ash, metals.
- Hazardous Waste: Radioactive materials, explosives.
- Biomedical Waste: Bandages, needles, medicines.
- Electronic Waste (E-Waste): Old phones, computers.
Biodegradable vs Non-Biodegradable Waste:
| Feature | Biodegradable Waste | Non-Biodegradable Waste |
|---|---|---|
| Definition | Easily decomposed by microbes. | Takes a long time to degrade. |
| Examples | Vegetable peels, paper. | Plastics, metals. |
Necessity of Waste Management:
- Reduces pollution and conserves resources.
- Generates employment opportunities.
- Enhances public health and environmental quality.
Methods of Solid Waste Management
- Segregation: Sorting waste into wet and dry categories.
- Recycling: Transforming waste materials into reusable products.
- Composting: Using organic waste to produce manure.
- Incineration: Burning waste to generate energy.
- Landfilling: Safe disposal of non-recyclable waste.
Common Question: Why is segregation important in waste management?
It ensures effective recycling and reduces the burden on landfills.
Principles of Waste Management
- Reduce: Minimize waste generation by limiting resource usage.
- Reuse: Use materials like bags and containers multiple times.
- Recycle: Convert waste materials like paper and plastic into new products.
- Rethink: Change consumption habits to reduce waste.
- Refuse: Avoid non-eco-friendly products like plastic bags.
Natural Disasters and Disaster Management
Types of Disasters:
- Natural Disasters: Earthquakes, floods, cyclones.
- Man-Made Disasters: Industrial accidents, fires, chemical leaks.
Steps in Disaster Management:
- Preparation: Awareness campaigns and infrastructure planning.
- Mitigation: Reducing disaster impact through technology and planning.
- Relief: Providing first aid, food, and shelter.
- Rehabilitation: Restoring affected areas and rebuilding.
First Aid Tips:
- For burns: Rinse with cold water.
- For fractures: Immobilize with a splint.
Role of Individuals in Environmental Management
- Use eco-friendly products.
- Conserve water and energy.
- Participate in tree-planting drives.
- Spread awareness about waste management.
Innovative Waste Management Practices
- Vermicomposting: Using earthworms to decompose organic waste.
- Bio-Gas Plants: Converting wet waste into methane for energy.
- E-Waste Recycling: Extracting metals from old electronics.
Common Question: What is the role of biogas plants in waste management?
They provide renewable energy while reducing organic waste.
Conclusion:
Environmental management is crucial for achieving sustainable development. By adopting practices like solid waste management, disaster preparedness, and resource conservation, we can protect the planet for future generations. This chapter highlights the importance of individual and collective efforts in preserving the environment.
Information Communication Technology: The New Direction of Progress
Information Communication Technology: The New Direction of Progress chapter focuses on the transformative role of Information Communication Technology (ICT) in our lives. ICT involves devices, systems, and technologies used to collect, share, process, and manage information. From early computers to modern supercomputers, this chapter highlights the evolution of technology, its applications in science, and the opportunities it offers in education, research, and industries.
What is Information Communication Technology (ICT)?
- Definition: ICT refers to the use of devices and systems for gathering, sharing, processing, and storing information.
- Examples: Computers, mobile phones, radios, televisions, and the internet.
Importance:
- Facilitates rapid information exchange.
- Supports decision-making processes in science, business, and governance.
- Bridges communication gaps globally.
Common Question: Why is ICT crucial in today’s world?
ICT drives advancements in every field, from healthcare and education to research and communication.
Generations of Computers
First Generation (1946–1959):
- Used vacuum tubes.
- Large in size and consumed high power.
- Example: ENIAC.
Second Generation (1959–1965):
- Used transistors, replacing vacuum tubes.
- Smaller, faster, and more reliable.
Third Generation (1965–1971):
- Used integrated circuits (ICs).
- Introduced operating systems for multitasking.
Fourth Generation (1971–Present):
- Used microprocessors.
- Led to personal computers (PCs).
Fifth Generation (Present and Beyond):
- Uses artificial intelligence (AI).
- Advanced technologies like quantum computing and machine learning.
Components of a Computer
- Input Unit: Devices like keyboards and mice used to feed data into the computer.
- Processing Unit:
- Memory Unit: Stores data temporarily (RAM) or permanently (ROM).
- Control Unit: Directs operations within the computer.
- ALU (Arithmetic Logic Unit): Performs calculations and logical operations.
- Output Unit: Displays results using monitors, printers, or speakers.
Types of Software
- System Software: Manages hardware and software resources (e.g., operating systems like Windows, Linux).
- Application Software: Designed for specific tasks (e.g., MS Word, Excel).
Example Activity: Use MS Excel to create graphs comparing data points.
ICT Devices and Their Applications
| Device | Function | Applications |
|---|---|---|
| Computer/Laptop | Data processing and storage. | Education, research, and business. |
| Mobile Phone | Communication and information access. | Personal and professional use. |
| Radio | Audio broadcasting. | News, music, and emergency alerts. |
| Television | Audio-visual broadcasting. | Education and entertainment. |
Common Question: What makes computers the most versatile ICT device?
Their ability to perform diverse tasks like data analysis, programming, and multimedia editing.
Role of ICT in Science and Technology
- Prediction: Enables meteorological forecasts and climate studies.
- Demonstration: Simplifies complex concepts through animations and simulations.
- Example: Animation of the human nervous system.
- Research: Provides access to global data repositories via the internet.
Common Question: How does ICT benefit scientific research?
ICT accelerates data collection, analysis, and sharing across global platforms.
Internet Applications in ICT
- Search Engines: Tools like Google and Bing for accessing global information.
- Email and Messaging: Instant communication across the globe.
- Video Conferencing: Real-time virtual meetings, enhancing collaboration.
- Educational Platforms: Online courses and resources (e.g., Khan Academy, Coursera).
Using ICT Tools for Learning
Microsoft Word:
- Used for creating documents.
- Features: Formatting text, inserting tables, and adding images.
Microsoft Excel:
- Used for data organization and analysis.
- Features: Calculations, graph creation, and data visualization.
Microsoft PowerPoint:
- Used for presentations.
- Features: Slide animations, transitions, and multimedia integration.
Activity: Create a presentation on “Applications of ICT in Healthcare.”
Challenges in ICT
- Digital Divide: Unequal access to technology across regions.
- Cybersecurity Threats: Risks of hacking, phishing, and data theft.
- Overdependence: Excessive reliance on ICT can reduce critical thinking skills.
Common Question: How can we ensure responsible use of ICT?
By practicing cybersecurity measures and maintaining a balance between digital and offline activities.
Opportunities in the Field of ICT
- Software Development: Application and operating system design.
- Hardware Engineering: Development and maintenance of physical components.
- Data Analysis: Interpreting large datasets for businesses and research.
- ICT Training: Teaching new users to operate ICT systems effectively.
Common Question: What career opportunities exist in ICT for students?
From coding and app development to digital marketing and AI research, ICT offers a wide range of career paths.
Conclusion:
The chapter “Information Communication Technology: The New Direction of Progress” highlights the revolutionary impact of ICT on education, communication, and research. By understanding and utilizing ICT tools, students can enhance their learning and contribute to technological advancements. This knowledge opens doors to numerous opportunities in the ever-evolving digital world.
Reflection of Light
Reflection of Light chapter explores the behavior of light when it strikes surfaces, the formation of images through reflection, and the principles underlying mirrors. It covers the laws of reflection, types of mirrors, and applications of concave and convex mirrors. This chapter provides the foundation for understanding optical phenomena and the practical use of reflective surfaces in daily life and technology.
What is Light?
- Definition: Light is a form of electromagnetic radiation that enables us to see objects.
- Significance: Light allows us to perceive the world and observe phenomena like sunrise, rainbows, and starlight.
Common Question: How does light help us see objects?
Objects reflect light into our eyes, forming images on the retina, which the brain interprets.
Reflection of Light
- Definition: The phenomenon of light bouncing back from a surface.
- Types of Reflection:
- Regular Reflection: Occurs on smooth surfaces, forming clear images.
- Irregular Reflection: Occurs on rough surfaces, scattering light in different directions.
Example: A polished metal plate provides regular reflection, while a piece of paper causes irregular reflection.
Laws of Reflection
- The angle of incidence equals the angle of reflection.
- The incident ray, reflected ray, and normal lie in the same plane.
Common Question: Why do mirrors provide clear images?
Smooth surfaces cause regular reflection, ensuring reflected rays form coherent images.
Types of Mirrors
a. Plane Mirrors
- Characteristics:
- Produces virtual, erect images.
- The image size equals the object size.
Application: Used in household mirrors and periscopes.
b. Spherical Mirrors
- Concave Mirror: The inner surface reflects light.
- Uses: Dental mirrors, telescopes, and solar devices.
- Convex Mirror: The outer surface reflects light.
- Uses: Rear-view mirrors in vehicles.
Terms Related to Spherical Mirrors:
- Pole (P): The center of the mirror’s surface.
- Centre of Curvature (C): The center of the sphere from which the mirror is derived.
- Principal Axis: The line joining the pole and center of curvature.
- Focal Length (f): The distance between the pole and principal focus.
Image Formation by Plane and Spherical Mirrors
Plane Mirrors:
- Images are virtual, erect, and laterally inverted.
- The distance of the image from the mirror equals the object’s distance.
Example Activity: Place an object in front of a plane mirror to observe lateral inversion.
Concave Mirrors:
- Form real, inverted, or virtual, erect images depending on the object’s position.
- Applications: Shaving mirrors, solar concentrators.
Convex Mirrors:
- Always form virtual, erect, and diminished images.
- Applications: Security and rear-view mirrors.
Cartesian Sign Conventions
- Object Distance (u): Negative when measured to the left of the mirror.
- Image Distance (v): Positive for real images and negative for virtual images.
- Focal Length (f): Negative for concave mirrors and positive for convex mirrors.
Mirror Formula:
Magnification (M):
Example Question: How do we calculate the size of an image?
Use the magnification formula: M=h2/h1
Applications of Reflection
- Solar Cookers: Uses concave mirrors to concentrate sunlight.
- Street Lamps: Convex mirrors spread light over a large area.
- Optical Instruments: Microscopes and telescopes use reflection principles.
Activity: Demonstrate focusing sunlight with a concave mirror to observe heat concentration.
Practical Examples and Problem-Solving
Example:
An object 5 cm high is placed 20 cm from a concave mirror with a focal length of 10 cm. Find the image distance and size.
- Solution:
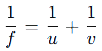
Substituting f=−10 cm,u=−20
v=−20cm (real and inverted image).
Conclusion:
The chapter “Reflection of Light” provides insights into the behavior of light when it interacts with surfaces. By understanding mirrors and their applications, students can appreciate optical technologies’ role in daily life and scientific innovations. Mastery of this chapter equips learners with foundational knowledge in optics, paving the way for advanced studies.
Study of Sound
Study of Sound chapter explains the nature of sound as a wave, its properties, how it travels through various mediums, and its applications in science and daily life. It covers concepts such as sound waves, their propagation, velocity, reflection, and practical uses like SONAR and sonography. Additionally, it introduces the structure of the human ear and its role in hearing.
Nature of Sound
- Definition: Sound is a form of energy that creates a sensation of hearing.
- Wave Nature: Sound travels as a longitudinal wave, involving compressions (high density) and rarefactions (low density).
- Medium Requirement: A medium like air, water, or solid is necessary for the propagation of sound.
Common Question: Why can’t sound travel in a vacuum?
Sound requires a medium to transfer energy through particle vibrations, which are absent in a vacuum.
Types of Waves
- Longitudinal Waves: Particles oscillate parallel to the wave’s direction. Example: Sound waves.
- Transverse Waves: Particles oscillate perpendicular to the wave’s direction. Example: Water waves.
Properties of Sound Waves
- Wavelength (λ): Distance between two consecutive compressions or rarefactions.
- Frequency (f): Number of oscillations per second, measured in Hertz (Hz).
- Amplitude: Maximum displacement of particles; determines loudness.
- Time Period (T): Time taken for one oscillation.
5. Velocity (v): Speed of the wave through a medium.
v=fλ
Example Activity: Use a tuning fork to observe sound vibrations in water.
Velocity of Sound
- Formula: v=λ×f
- Dependence on Medium:
- Faster in solids, slower in liquids, and slowest in gases.
- Increases with temperature in gases.
Table: Velocity of sound in various mediums at 25°C:
| Medium | Velocity (m/s) |
|---|---|
| Aluminum (solid) | 6420 |
| Water (liquid) | 1498 |
| Air (gas) | 346 |
Audible and Inaudible Sounds
- Audible Range: 20 Hz to 20,000 Hz (human hearing range).
- Infrasound: Frequency below 20 Hz (e.g., vibrations before an earthquake).
- Ultrasound: Frequency above 20,000 Hz (e.g., used by bats and dolphins).
Common Question: Why can dogs hear ultrasonic sounds?
Dogs have a wider frequency range of hearing, enabling them to detect ultrasounds.
Reflection of Sound
- Definition: Sound bounces back from a solid or liquid surface.
- Laws of Reflection:
- Angle of incidence equals the angle of reflection.
- Incident wave, reflected wave, and normal lie in the same plane.
Applications:
- Echolocation by bats and dolphins.
- SONAR in ships for underwater detection.
Echo and Reverberation
Echo:
- Definition: Repetition of sound due to reflection from a distant surface.
- Condition: Minimum distance for echo at 22°C is 17.2 meters.
Reverberation:
- Definition: Prolonged sound due to multiple reflections.
- Prevention: Use of sound-absorbing materials like curtains and carpets.
SONAR (Sound Navigation and Ranging)
- Definition: A technique using ultrasonic waves to detect objects underwater.
- Working:
- Ultrasonic waves are sent into water.
- Reflected waves from objects are received.
- Distance is calculated using:
Applications:
- Measuring sea depth.
- Locating submarines and sunken ships.
Human Ear
- Parts:
- Outer Ear (Pinna): Collects sound waves.
- Middle Ear: Amplifies vibrations through the eardrum and three small bones.
- Inner Ear (Cochlea): Converts vibrations into electrical signals sent to the brain.
Common Question: Why should we avoid loud music?
Excessive noise can damage the eardrum and impair hearing.
Applications of Ultrasound
- Medical Imaging:
- Used in sonography to visualize internal organs and monitor fetal growth.
- Industrial Use:
- Detecting cracks in metal.
- Cleaning intricate machine parts.
- Sterilization:
- Killing bacteria in liquids like milk.
Conclusion:
The chapter “Study of Sound” provides an in-depth understanding of sound waves, their properties, and practical applications. From SONAR and medical diagnostics to communication and entertainment, sound plays a crucial role in technology and daily life. Mastery of these concepts helps students appreciate the scientific principles behind sound-related phenomena.
Carbon - An Important Element
Carbon – An Important Element chapter focuses on the properties, occurrence, and importance of carbon in nature. It highlights the versatile bonding properties of carbon that allow it to form a wide variety of compounds. The chapter covers the allotropes of carbon, hydrocarbons, and carbon dioxide, emphasizing its significance in life processes and industrial applications. Students will also explore the eco-friendly uses of carbon, such as in biogas and fire extinguishers.
Properties of Carbon
- Symbol: C
- Atomic Number: 6
- Atomic Mass: 12
- Electronic Configuration: 2, 4
- Valency: 4 (tetravalent)
- Nature: Non-metal.
Key Characteristics:
- Forms covalent bonds by sharing electrons.
- Can form single, double, or triple bonds with other carbon atoms or elements.
- Found in free (diamond, graphite) and combined states (compounds like CO2_22, carbohydrates, and hydrocarbons).
Allotropy of Carbon
Definition:
Allotropy refers to the existence of an element in two or more different forms in the same physical state, with different physical properties but identical chemical properties.
Crystalline Allotropes:
Diamond:
- Structure: Each carbon atom is covalently bonded to four others in a tetrahedral arrangement, forming a rigid 3D structure.
- Properties:
- Hardest natural substance.
- High melting point (3500°C).
- Does not conduct electricity due to the absence of free electrons.
- Uses:
- Cutting tools for glass and rocks.
- Jewelry.
- Knives for eye surgery.
Graphite:
- Structure: Carbon atoms form hexagonal layers with weak forces between layers, allowing them to slide over each other.
- Properties:
- Soft and slippery.
- Good conductor of electricity due to free electrons.
- Uses:
- Electrodes.
- Pencils.
- Lubricants.
Fullerene:
- Structure: Molecules shaped like spheres (buckyballs) or tubes (buckytubes).
- Properties:
- Lightweight.
- Exhibits superconductivity at low temperatures.
- Uses:
- Insulators.
- Water purification catalysts.
Amorphous Allotropes:
- Examples: Coal, charcoal, coke.
- Uses: Fuels, reducing agents, water purification.
Hydrocarbons
- Definition: Organic compounds made up of only carbon and hydrogen.
- Types:
- Saturated Hydrocarbons: Contain single bonds (e.g., methane, ethane).
- Unsaturated Hydrocarbons: Contain double or triple bonds (e.g., ethene, ethyne).
Example Reaction:
Methane formation:
CH4+2O2→CO2+2H2O+Heat
Activity: Observe the bluish flame when methane burns.
Carbon Dioxide
- Formula: CO2
- Occurrence: Found in the atmosphere (~0.03%) and as a product of respiration and combustion.
- Properties:
- Colorless and odorless gas.
- Denser than air.
Chemical Properties:
- Reacts with limewater to form calcium carbonate:
Ca(OH)2+CO2→CaCO3+H2O (Turns limewater milky.)
Uses:
- Carbonated beverages.
- Fire extinguishers.
- Dry ice for cooling.
Methane
- Formula: CH4
- Occurrence: Found in natural gas, biogas, and marshes.
- Properties:
- Highly flammable.
- Produces less CO2 upon combustion.
Uses:
- Domestic and industrial fuel.
- Production of organic compounds like ethanol.
Biogas
- Definition: Gas produced by the anaerobic decomposition of organic matter.
- Composition: 55-60% methane, CO2, and traces of hydrogen.
- Process:
- Organic matter decomposes into acids.
- Methanogenic bacteria convert acids into methane.
Uses:
- Cooking gas.
- Generating electricity.
Carbon in Daily Life
- Food: Carbohydrates, proteins, and fats.
- Fuel: Coal, petroleum, and natural gas.
- Fibers: Cotton, wool, silk.
Environmental Impact of Carbon Compounds
- Fossil Fuels: Combustion releases CO2, contributing to global warming.
- Deforestation: Reduces carbon sequestration.
Conclusion:
Carbon is a versatile element, forming the backbone of life and numerous industrial applications. Its unique properties, such as catenation and allotropy, enable it to form a wide variety of compounds, from simple hydrocarbons to complex biomolecules. Understanding its role and sustainable usage is vital for progress and environmental conservation.
Substances in Common Use
Substances in Common Use chapter explores the various substances we encounter in our daily lives, such as soaps, detergents, plastics, glass, and acids. It discusses their composition, properties, manufacturing processes, and uses. The chapter also delves into the environmental impacts of certain substances and suggests sustainable alternatives to minimize harm. By understanding these topics, students gain insight into the science behind everyday materials.
Acids, Bases, and Salts
- Acids: Substances that release hydrogen ions (H+) in water.
- Examples: Hydrochloric acid (HCl), sulfuric acid (H2SO4H).
- Bases: Substances that release hydroxide ions (OH−) in water.
- Examples: Sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)2).
- Salts: Compounds formed by the neutralization of an acid and a base.
- Examples: Sodium chloride (NaCl), calcium carbonate (CaCO3).
Properties:
- Acids are sour and turn blue litmus red.
- Bases are bitter and slippery, turning red litmus blue.
- Salts can be neutral, acidic, or basic depending on their parent acid and base.
Soaps and Detergents
Soaps: Sodium or potassium salts of fatty acids.
- Example: Sodium stearate.
- Process of Saponification:
- Fats and oils react with alkali to form soap and glycerol.
- Equation: Fat+NaOH→Soap+Glycerol
Detergents: Synthetic cleansing agents effective in hard water.
- Example: Sodium lauryl sulfate.
Comparison:
| Feature | Soap | Detergent |
|---|---|---|
| Natural/Synthetic | Natural | Synthetic |
| Hard Water Usage | Ineffective | Effective |
Common Question: Why are detergents preferred over soaps in hard water?
Detergents do not form scum with calcium and magnesium ions, making them effective in hard water.
Plastics and Polymers
- Definition: Plastics are synthetic polymers made from monomers.
- Examples: Polyethylene, polystyrene.
- Types of Plastics:
- Thermoplastics: Can be reshaped by heating (e.g., PVC, polyethylene).
- Thermosetting Plastics: Cannot be reshaped after setting (e.g., bakelite, melamine).
Environmental Impact:
- Non-biodegradable; contributes to pollution.
- Alternatives: Biodegradable plastics and recycling.
Glass
- Composition: Silica (SiO2), sodium carbonate (Na2CO3), and calcium carbonate (CaCO3).
- Types of Glass:
- Soda-lime Glass: Used in windows and bottles.
- Borosilicate Glass: Heat-resistant; used in laboratory equipment.
- Fused Quartz: High thermal stability; used in lenses.
Process of Glass Making:
- Mixing raw materials.
- Heating in a furnace.
- Cooling and shaping.
Fibers
- Natural Fibers: Derived from plants and animals. Examples: Cotton, wool.
- Synthetic Fibers: Made from petrochemicals. Examples: Nylon, polyester.
Properties of Synthetic Fibers:
- Durable, elastic, resistant to water and stains.
- Environmental Concern: Non-biodegradable.
Fertilizers
- Definition: Substances that supply nutrients to plants.
- Types:
- Organic Fertilizers: Compost, manure.
- Chemical Fertilizers: Urea
(CO(NH2)2), NPK (Nitrogen, Phosphorus, Potassium).
Impact of Overuse:
- Soil degradation.
- Water pollution through eutrophication.
Alternatives: Bio-fertilizers like Rhizobium.
Cement
- Composition: Limestone (CaCO3), silica (SiO2), alumina (Al2O3).
- Uses: Construction of buildings, roads, and dams.
- Process:
- Mixing raw materials.
- Heating in a rotary kiln.
- Grinding into fine powder.
Chemicals in Food
- Preservatives: Prevent spoilage (e.g., sodium benzoate).
- Artificial Sweeteners: Saccharin, aspartame.
- Flavor Enhancers: MSG (Monosodium Glutamate).
Concerns: Overuse may cause health issues.
Conclusion:
This chapter emphasizes the importance of understanding the substances we use daily, their advantages, and their environmental impacts. By adopting sustainable alternatives and responsible usage, we can minimize harm to the environment while benefiting from these substances. Understanding their properties and applications helps students appreciate the science behind common materials.
Life Processes in Living Organisms
Life Processes in Living Organisms chapter explores the essential biological processes that occur in living organisms to sustain life. These processes include respiration, photosynthesis, digestion, excretion, and reproduction. Each process is intricately linked to maintaining homeostasis and supporting growth, development, and survival. Understanding these life processes helps us appreciate the complexity and functionality of living organisms.
What are Life Processes?
- Definition: Life processes are biological activities essential for the maintenance and continuation of life.
- Examples: Respiration, digestion, excretion, and reproduction.
Importance of Life Processes:
- Provide energy for growth and repair.
- Maintain internal balance (homeostasis).
- Enable reproduction and survival of species.
Nutrition
- Definition: The process of obtaining and utilizing food for energy and growth.
- Types of Nutrition:
- Autotrophic Nutrition: Organisms produce their food (e.g., plants via photosynthesis).
- Equation:
6CO2+6H2O+Sunlight→C6H12O6+6O2
- Equation:
- Heterotrophic Nutrition: Organisms depend on others for food (e.g., animals, fungi).
- Types:
- Herbivores (plant-eaters).
- Carnivores (meat-eaters).
- Omnivores (both plant and meat-eaters).
- Types:
- Autotrophic Nutrition: Organisms produce their food (e.g., plants via photosynthesis).
Respiration
- Definition: The process of breaking down glucose to release energy.
- Types of Respiration:
- Aerobic Respiration: Occurs in the presence of oxygen.
- Equation:
C6H12O6+6O2→6CO2+6H2O+Energy (ATP)
- Equation:
- Anaerobic Respiration: Occurs in the absence of oxygen.
- Byproducts: Lactic acid (in muscles) or ethanol and CO₂ (in yeast).
- Aerobic Respiration: Occurs in the presence of oxygen.
Common Question: Why do we feel fatigued after intense exercise?
During vigorous exercise, oxygen supply is insufficient, leading to anaerobic respiration and lactic acid buildup, causing fatigue.
Transportation in Living Organisms
Definition: The movement of substances like nutrients, gases, and waste products within the organism.
In Animals:
- Circulatory System: Blood transports oxygen, nutrients, and waste.
- Components: Heart, blood vessels, and blood.
In Plants:
- Xylem: Transports water and minerals from roots to leaves.
- Phloem: Transports food from leaves to other parts of the plant.
Common Question: How is transportation in plants different from animals?
Plants rely on passive mechanisms like transpiration pull, while animals use an active circulatory system powered by the heart.
Excretion
Definition: The process of removing metabolic waste from the body.
In Humans:
- Organs: Kidneys, ureters, urinary bladder, and urethra.
- Process: Blood is filtered in the kidneys, and waste is excreted as urine.
In Plants:
- Excrete oxygen as a byproduct of photosynthesis.
- Other waste is stored in vacuoles or shed with leaves.
Reproduction
- Definition: The biological process by which organisms produce offspring.
- Types of Reproduction:
- Asexual Reproduction: Involves one parent, producing genetically identical offspring (e.g., binary fission in bacteria).
- Sexual Reproduction: Involves two parents, producing genetically diverse offspring (e.g., humans, flowering plants).
Common Question: Why is reproduction not essential for individual survival?
Reproduction ensures species continuity but does not directly affect an individual’s survival.
Control and Coordination
- Definition: The ability of an organism to respond to stimuli.
- In Animals:
- Nervous System: Brain, spinal cord, and nerves transmit signals.
- Hormonal System: Hormones regulate growth and metabolism.
- In Plants:
- Tropic movements (e.g., phototropism) in response to light, water, and gravity.
Growth and Development
- Definition: Increase in size and complexity of an organism over time.
- Types:
- Isometric Growth: Equal growth in all dimensions (e.g., bacteria).
- Allometric Growth: Unequal growth of body parts (e.g., humans).
Regulation of Internal Environment (Homeostasis)
- Definition: Maintaining a stable internal environment despite external changes.
- Examples:
- Sweating to cool the body.
- Regulation of blood sugar by insulin.
Conclusion:
The chapter “Life Processes in Living Organisms” highlights the essential biological mechanisms that ensure survival, growth, and reproduction. By understanding these processes, students gain insights into how organisms adapt to their environment and sustain life. This knowledge forms the foundation of biology and its applications in healthcare, agriculture, and environmental science.
Heredity and Variation
Heredity and Variation chapter explains how traits are passed from one generation to the next and the differences that arise between individuals of the same species. It focuses on genetic inheritance, Mendel’s experiments, chromosomal behavior, and the significance of variation in evolution. The chapter provides an in-depth understanding of genes, alleles, and the principles governing heredity and variation.
What is Heredity and Variation?
- Heredity: The transmission of genetic traits from parents to offspring.
- Variation: Differences in traits among individuals of the same species.
Mendel’s Experiments
Gregor Mendel:
- Known as the Father of Genetics.
- Conducted experiments on pea plants to study inheritance.
Why Pea Plants?
- Easy to cultivate.
- Short lifecycle.
- Clear contrasting traits (e.g., tall vs. dwarf, yellow vs. green seeds).
Key Findings:
- Traits are controlled by “factors” (now called genes).
- Each trait has two alleles (dominant and recessive).
- Traits are inherited independently.
Mendel’s Laws of Inheritance
Law of Segregation:
- Each organism carries two alleles for a trait, which segregate during gamete formation.
- Example: A tall plant (Tt) produces gametes carrying either T or t.
Law of Independent Assortment:
- Alleles of different traits are inherited independently.
- Example: Seed color (yellow or green) and seed shape (round or wrinkled) are inherited independently.
Genetic Terminology
- Gene: A unit of heredity located on chromosomes.
- Allele: Variants of a gene controlling the same trait.
- Dominant Trait: Expressed in the presence of one or two dominant alleles.
- Recessive Trait: Expressed only in the absence of a dominant allele.
- Genotype: Genetic makeup of an organism (e.g., TT, Tt).
- Phenotype: Observable traits (e.g., tall plant).
Monohybrid Cross
- A genetic cross involving one pair of contrasting traits.
Example:
Cross between tall (TT) and dwarf (tt) pea plants:
- F1 Generation: All tall (Tt).
- F2 Generation: 3:1 phenotypic ratio (Tall:Dwarf).
Dihybrid Cross
- A genetic cross involving two pairs of contrasting traits.
Example:
Cross between plants with yellow round seeds (YYRR) and green wrinkled seeds (yyrr):
- F2 Generation Phenotypic Ratio: 9:3:3:1
Sex Determination
- In Humans:
- Chromosomal basis:
- XX: Female
- XY: Male
- The father’s sperm determines the offspring’s sex.
- Chromosomal basis:
Common Question: Why is the father responsible for determining the child’s sex?
The father contributes either an X or a Y chromosome, while the mother always contributes an X chromosome.
Chromosomal Behavior
- Chromosomes: Structures carrying genetic material.
- Homologous Chromosomes: A pair of chromosomes, one from each parent, carrying genes for the same traits.
- Mutation: Sudden change in the DNA sequence, leading to variation.
Genetic Disorders
Sickle Cell Anemia:
- Cause: Mutation in the hemoglobin gene.
- Effect: Abnormal red blood cell shape.
Down Syndrome:
- Cause: An extra chromosome 21.
- Effect: Developmental delays and physical abnormalities.
Hemophilia:
- Cause: Recessive gene on the X chromosome.
- Effect: Blood fails to clot properly.
Variation and Evolution
Sources of Variation:
- Mutations.
- Genetic recombination during meiosis.
- Crossing over during gamete formation.
Significance of Variation:
- Enables adaptation to changing environments.
- Drives evolution through natural selection.
Applications of Genetics
- Agriculture: Development of high-yield crops.
- Medicine: Gene therapy and personalized medicine.
- Forensics: DNA fingerprinting for crime investigations.

